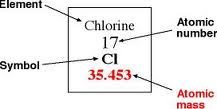
With this blog we did book work about Quantum Mechanics. Quantum Mechanics states that only a certain amount of electrons can be in a shell. The number increases as you continue out from the nucleus. With the first shell it can only hold two electrons, the second shell has eight, the third shell has 18. The levels or shells are also called orbitals. These orbitals have sub-levels starting after the first orbital. Each level starts with the S orbital. The S orbital holds two electrons. When the S orbital is filled, you start to fill the sub-levels. The first sub-level is the P. Did you know you can name elements by their orbitals. An example of electron configurations is Bromine (Br) Bromine has 35 electrons: [Ar]4s23d104p5.
This is my first set of questions:
This is my second set of questions: