We should first understand the models of elements we have Daltons atomic theory that explains the all matter is small and the particles are called atoms.
The second is J.J. Thomson's plum pudding model that proposed that negatively charged elections were distributed throughout a uniform positive charge.
The last is Ernest Rutherford. He expected most of the fast moving and relatively massive alpha particles to pass straight through gold foil. He also expected a few of the alpha particles to be slightly deflected by the electron in the gold atoms.
Remember back in elementary school when we were first learning about atoms and they told you about neutrons being neutral, protons being positive and electrons being negative. Yes that is true but now we go even more in depth of that. Now we learned about Isotopes and atomic mass. Crazy to think that such a little thing has a lot of depth to it.
You have protons and neutrons in the nucleus of an atom and the electrons are floating around the nucleus of the atom.
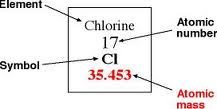
The final steps to understand an atom are as followed:
- You look at the atomic number and the the atomic number is the same number of protons and electrons.
- Mass number is the average of all isotopes of the element.
- To find neurons you subtract the Atomic number form the atomic mass
So understanding atomic structure isn't as hard as it sounds.




No comments:
Post a Comment