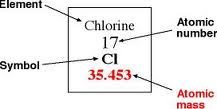
- Worksheets
- Elements and Such
- Conductivity Lab
- Into to Ionic bonding and Ionic Compound Formulas
- Magnesium Lab
- Covalent Bonds
- Types of Reaction
What is important about it:
Each blog I wrote was very interesting and fun. The class was just engaging to be in. I enjoyed learning about the elements, the magnesium lab and the types of reaction lab. The blogs and book work gave me more knowledge of Chemistry.
Applying:
The only reason I apply chemistry to life everyday is because I have it 1st hour. I guess I could use chemistry everyday somehow.
Evaluating:
This 2nd quarter has gone by fast but I think I did my best. My blogs were really interesting, in-depth, and detailed. Most has really good references and youtube videos that enhanced the blog and my showed my knowledge of the subject I'm talking about. So I would give myself an A for effort.
Creating:
Form the 1st quarter I think I reached my goal of putting more visual effects into my blog and using more technology in the class. I also have reached my goal if having deeper explanations of what we were studying. This next quarter I would like to get better and writing blogs and getting more 4's. For this next quarter my goal is to get all 4's.
Evaluating:
This 2nd quarter has gone by fast but I think I did my best. My blogs were really interesting, in-depth, and detailed. Most has really good references and youtube videos that enhanced the blog and my showed my knowledge of the subject I'm talking about. So I would give myself an A for effort.
Creating:
Form the 1st quarter I think I reached my goal of putting more visual effects into my blog and using more technology in the class. I also have reached my goal if having deeper explanations of what we were studying. This next quarter I would like to get better and writing blogs and getting more 4's. For this next quarter my goal is to get all 4's.